Acknowledgement
This guidance uses the terms ‘woman’ and ‘mother,’ which are intended to be inclusive of anyone who may use other self-identifying terms and aims to encompass all for whom this guidance is relevant.
Consumer Engagement Statement
All interactions between health care staff with consumers (women, mothers, patients, carers and families) should be undertaken with respect, dignity, empathy, honesty and compassion.
Health care staff should actively seek and support consumer participation and collaboration to empower them as equal partners in their care.
Definitions
Chronic hypertension occurring in pregnancy (Essentialand secondary) | - Hypertension diagnosed prior to conception or prior to 20+0 weeks
- Includes women entering pregnancy on antihypertensive therapy
- Essential hypertension (no secondary cause determined)
- Secondary hypertension where causes may include:
- renal parenchymal disease (e.g., glomerulonephritis, reflux nephropathy and adult polycystic kidney disease)
- renal artery stenosis
- systemic disease with renal involvement (e.g., diabetes mellitus)
- systemic lupus erythematosus (SLE))
- endocrine disorders (e.g., phaeochromocytoma, Cushing’s syndrome, primary hyperaldosteronism, hyper- or hypothyroidism and acromegaly)
- coarctation of the aorta
- obstructive sleep apnoea
- medications or supplements. (e.g., oral contraceptives, nonsteroidal anti-inflammatory drugs, corticosteroids, cocaine, stimulants, antipsychotic medications).
|
|---|
| White Coat Hypertension | - Hypertension characterised by an elevated BP in a clinical setting and a normal BP at other times.
- Typically diagnosed by 24-hour ambulatory BP monitoring or home BP monitoring using an appropriately validated device.
- Women with white coat hypertension are at an increased risk of developing pre-eclampsia, compared with normotensive women.
|
|---|
| Masked hypertension | - Hypertension characterised by a normal BP in a clinical setting and an elevated BP at other times.
- Typically diagnosed by 24-hour ambulatory BP monitoring or home BP monitoring using an appropriately validated device.
|
|---|
Hypertension in pregnancy (Gestational Hypertension) | - New onset of hypertension arising after 20 weeks gestation
- No additional maternal or fetal features of pre-eclampsia
- Resolves within 3 months postpartum.
|
|---|
| Severe hypertension in pregnancy | - Systolic blood pressure ≥ 170 mmHg and/or
- Diastolic blood pressure ≥ 110 mmHg.
|
|---|
| Pre-eclampsia | - A multi-system disorder characterised by hypertension and involvement of one or more other organ systems and/or the fetus
- Proteinuria is common but is not mandatory to make the diagnosis.
- See Appendix 3.
|
|---|
Pre-eclampsia superimposed on chronic hypertension | - New onset systemic features of pre-eclampsia after 20+0 weeks gestation to women with pre-existing hypertension.
|
|---|
| Eclampsia | - Onset of seizures in a woman with pre-eclampsia.
|
|---|
| HELLP | - A variant of severe pre-eclampsia including:
- haemolysis
- elevated liver enzymes
- low Platelet count (Thrombocytopenia).
|
|---|
| Note: The term PIH (pregnancy induced hypertension) is no longer used | |
|---|
Abbreviations
| BD | Twice daily |
|---|
| CTG | Cardiotocograph |
|---|
| DBP | Diastolic blood pressure |
|---|
| DIC | Disseminated intravascular coagulation |
|---|
| FBE | Full blood examination |
|---|
| FDIU | Fetal death in utero |
|---|
| FHR | Fetal heart rate |
|---|
| HBPM | Home blood pressure monitoring |
|---|
| HT | Hypertension |
|---|
| IM | Intramuscular |
|---|
| IOL | Induction of labour |
|---|
| IV | Intravenous |
|---|
| LFT | Liver function tests |
|---|
| OD | Daily |
|---|
| PCR | Protein creatinine ratio |
|---|
| RCT | Randomised Control Trial |
|---|
| SBP | Systolic blood pressure |
|---|
Screening and prevention of Hypertensive Disorders of Pregnancy
- Screen all women for risk of pre-eclampsia early in pregnancy.
- At a minimum, stratify risk based on maternal characteristics, medical and obstetric history.
- The use of a combined first trimester screen between 11 and 14 weeks for risk of early onset pre-eclampsia (combined maternal features, biomarkers (PLGF and PAPPA) and sonography (uterine artery PI) is conditionally recommended based on local availability and access to the required resources.
- Women with >2 moderate risk factors or one strong risk factor should be recommended prophylaxis. (Tables 1&2).
Prophylaxis
- Aspirin 150 mg* daily at bedtime.
- commence prior to 16 weeks’ gestation and cease after 34 weeks’ gestation
- Plus, calcium carbonate 1g orally daily.**
- if dietary calcium intake is inadequate i.e. less than 4 serves of dairy per day.
*This dose is recommended by SOMANZ based on RCT trial evidence in a selected population. The most clearly effective aspirin dose may be between 80-160mg and has yet to be clearly determined.
** Data around Calcium for prevention of pre-eclampsia is being reviewed and may be of less benefit than previously thought.
Table 1. Conditions identified as ‘High Risk’ for developing pre-eclampsia
- Previous hypertensive disorder during prior pregnancy
|
- Chronic kidney disease or kidney impairment
|
|
- Pre-existing chronic hypertension
|
- Pre-existing type 1 or type 2 diabetes mellitus
|
- Autoimmune disorders e.g., systemic lupus erythematosus, anti-phospholipid syndrome
|
Table 2. Conditions identified as ‘Moderate Risk’ for developing pre-eclampsia
- Advanced maternal age (>40)
|
|
|
- Family history of pre-eclampsia
|
- Interpregnancy interval of 10 or more years
|
- Assisted reproduction technologies
|
- Systolic blood pressure >130mmHg and/or diastolic blood pressure >80mmHg
|
Chronic hypertension or hypertension detected before 20 weeks gestation
- Women with chronic hypertension have an increased risk of:
- severe hypertension in the third trimester
- superimposed pre-eclampsia
- fetal growth restriction
- placental abruption
- premature birth
- stillbirth.
| Maternal assessment | - At booking, consider investigations for secondary causes of hypertension, in consultation with physicians.
- Women taking other antihypertensive medication pre-pregnancy, should change to methyldopa, labetalol or nifedipine.
- At subsequent visits after 20 weeks gestation, monitor for signs of superimposed pre-eclampsia. Take a history of pre-eclampsia. symptoms. (e.g. headache, visual disturbance, epigastric or right upper quadrant pain)
- Perform a general examination, including abdominal palpation (fetal lie, presentation, size) and neurological examination if required. (reflexes and clonus)
|
|---|
| Blood pressure measurement | - Where appropriate, home blood pressure monitoring (HBPM) with the use of a validated blood pressure device can be used.
- The use of HBPM should not replace the minimum recommended frequency of antenatal reviews, according to the woman’s parity and gestation.
- Compliance and technique with home blood pressure monitoring should be reassessed at each review, to ensure ongoing suitability.
- See Appendix 2.
|
|---|
| Proteinuria | - At each antenatal appointment assess for proteinuria. (Appendix 1.)
|
|---|
| Blood tests | - At booking:
- Measurement of serum electrolytes and creatinine.
- Ongoing
- Request a pre-eclampsia screen if there is a sudden increase in blood pressure or new proteinuria. (spot urine protein:creatinine ratio (PCR), full blood examination (FBE), urea, creatinine, electrolytes, liver function tests (LFT).
|
|---|
| Fetal assessment | - Dating scan to ensure accurate dates.
- Fetal assessment may include:
- Serial ultrasound scans performed 4 weekly after 28 weeks
- fetal biometry
- amniotic fluid assessment
- fetal umbilical artery Doppler studies.
- If clinically indicated, CTG if > 28 weeks gestation e.g., abnormal fetal growth, decreased fetal movement.
|
|---|
| Antihypertensives | - Consider antihypertensives when systolic blood pressure persistently exceeds 140 mmHg and/or diastolic pressure exceeds 90 mmHg.
- Blood pressure target is ≤135/85mmHg.
|
|---|
| Pre-eclampsia prophylaxis | |
|---|
| Collaborative care | - Frequent review by an Obstetrician/GP Obstetrician and by a physician familiar with the management of hypertension in pregnancy, is recommend.
- Early referral to Obstetric led care at a health service of Maternity Capability Level 4 or higher, as required.
- Depending on Health Service capability, women needing two antihypertensive agents during pregnancy to manage blood pressure should be considered for further management referral, if not already done.
|
|---|
| Timing of birth | See Timing of Birth. |
|---|
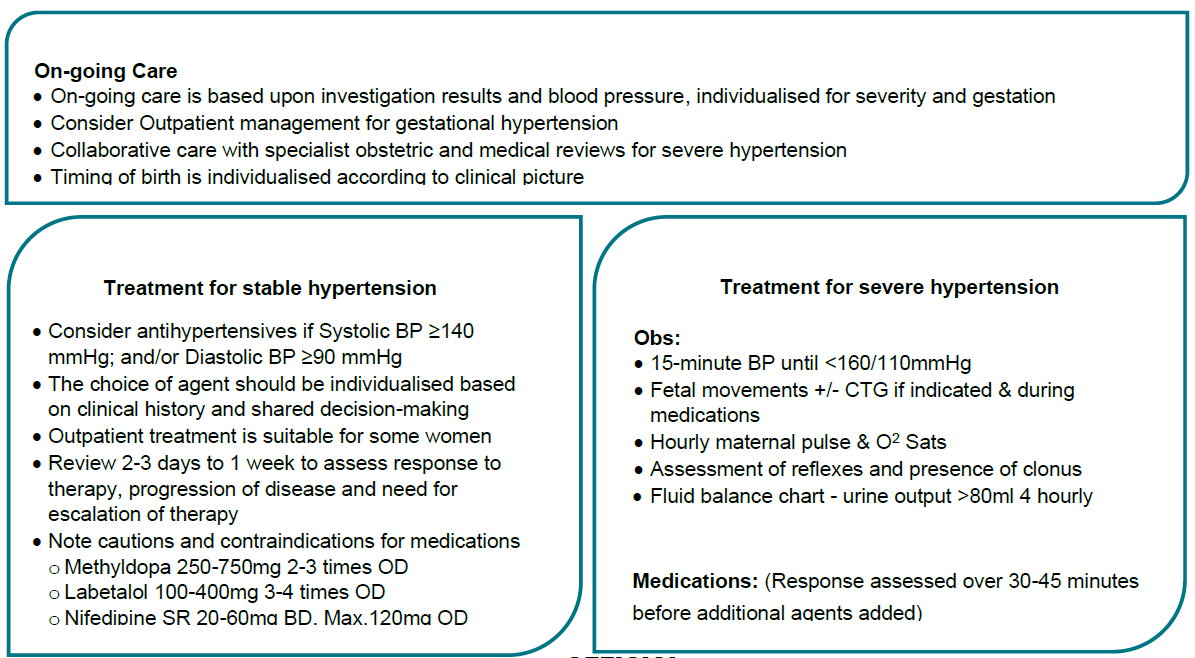
Hypertension diagnosed after 20 weeks gestation
| Maternal assessment | - Take a history of pre-eclampsia symptoms. (e.g., headache, visual disturbance, epigastric or right upper quadrant pain)
- General examination, including abdominal palpation. (fetal lie, presentation, size) and neurological examination. (reflexes and clonus)
|
|---|
| BP measurement | - Triage to severe or non-severe hypertension (SBP <160 mmHg and DBP <110 mmHg) by measuring BP over several hours.
- In consultation with the Obstetrician/GP Obstetrician, consider admission, day stay assessment or transfer with PIPER to a health service with appropriate capability.
- First line agents in pregnancy are methyldopa, labetalol or nifedipine. See Pharmacological Management for stable Hypertension.
- Blood pressure target is ≤135/85mmHg.
- Women presenting with new onset severe hypertension ≥160mmHg systolic or ≥110mmHg diastolic. (see severe HT)
|
|---|
| Proteinuria assessment | - Assess for proteinuria at each visit. (Appendix 1.)
- Absence of proteinuria does not exclude a diagnosis of pre-eclampsia.
- See Diagnosis of Pre-eclampsia for diagnostic criteria of pre-eclampsia. (Appendix 3.)
|
|---|
| Blood tests | - Consider performing bloods for Pre-eclampsia screen four weekly, or as indicated.
- FBE
- creatinine and U&E
- LFT’s
- coagulation profile only if evidence of thrombocytopenia or significant abnormalities in LFT’s.
|
|---|
| Additional investigations | - If features of pre-eclampsia are present, see ‘Pre-eclampsia’.
|
|---|
| Fetal assessment | - Fetal assessment may include:
- serial ultrasound scans performed 4 weekly after 28 weeks.
- fetal biometry
- amniotic fluid assessment
- fetal umbilical artery Doppler studies.
- If clinically indicated, CTG if > 28 weeks gestation e.g., abnormal fetal growth, decreased fetal movement.
|
|---|
| Ongoing Care | - Management after initial assessment will be based on results of investigations and ongoing blood pressure levels.
- Some women referred for assessment of new onset hypertension will have normal blood pressure and investigations. This could be ‘White Coat Hypertension’ and they may go on to develop Gestational Hypertension or/and Pre-eclampsia. Repeat assessment within 7 days.
Outpatient Care and Management - Outpatient management is usually suitable for women with gestational hypertension, without severe features.
- The timing and frequency of assessment will be determined by the gestation, the severity of disease and patient factors.
- An individualised care plan should be developed in the antenatal clinic by the obstetrician or GP obstetrician.
|
|---|
| Collaborative care | - Frequent review by an obstetrician/GP obstetrician and by a physician familiar with the management of hypertension in pregnancy, may be required.
- Early referral to obstetric led care at a health service of Maternity Capability Level 4 or higher, as required.
- Depending on the Health Service capability, women who require 2 or more antihypertensive agents to control blood pressure should be considered for referral for further management.
|
|---|
| Timing of birth | See Timing of birth |
|---|
Severe Hypertension
- Severe hypertension in pregnancy is defined as sustained blood pressure of ≥160/110mHg.
- Severe hypertension is a medical emergency and requires immediate assessment and treatment.
- Persistent severe hypertension in pregnancy is associated with poor maternal and fetal outcomes, which include haemorrhagic stroke, maternal death, eclampsia and placental abruption.
- Antihypertensive treatment should be commenced urgently in all women with a systolic blood pressure ≥160 mm Hg or a diastolic blood pressure ≥110 mmHg because of the risk of intracerebral haemorrhage.
- Pregnant women with hypertension may not appear ill, which may cause a delay in immediate care.
- Multidisciplinary care is required.
- Women with a diagnosis of severe pre-eclampsia, severe hypertension or increasing need for surveillance, requires hospital admission.
- Women with severe hypertension should be admitted to Birth Suite or other high dependency area for stabilisation and monitoring with 1:1 midwifery care and close fetal surveillance.
| Observations | - 15 minutely blood pressure until below <160/110 mmHg.
- Maternal report of fetal movements.
- Hourly Heart rate and Oxygen saturation.
- Neurological assessment of reflexes and presence of clonus.
- Fluid balance charted with review of urine output 4 hourly to ensure >80mL/4 hours.
|
|---|
| Investigations | Maternal: - Blood tests are ordered 2-3 times per week, depending on severity and rate of change in the woman’s condition
- FBE, U&E and LFT’s
- coagulation profile ONLY if platelets <100x109 or significant transaminase abnormality
- blood Group and Hold if planning birth
- urine PCR every 3-4 days, if proteinuria has not previously been detected.
Fetal: - Fetal heart rate or CTG from 28 weeks gestation, as indicated by the woman’s overall clinical condition.
- Ultrasound for fetal growth, amniotic fluid , UA Doppler. Frequency as clinically indicated.
|
|---|
| Treatment | - Refer to Pharmacological management for stable hypertension and Pharmacological management for severe hypertension.
|
|---|
| Antenatal corticosteroids | - Recommended from viability to 34+6 weeks gestation if birth is considered likely within the next 7 days
- Give 11.4 mg by intramuscular injection of betamethasone (Celestone Chronodose®) daily for two doses.
|
|---|
| Timing of birth | |
|---|
| Referral | - Minimum daily review by GPO, obstetric registrar or consultant.
- Notify paediatric team of all preterm antenatal inpatients. Paediatric consultation to discuss neonatal care, depending on gestation.
- Anaesthetic review if delivery is planned.
- If transfer is required, seek assistance from regional partners or PIPER.
|
|---|
| VTE | - Consider thromboprophylaxis with non-pharmacological and / or pharmacological measures during the antenatal and postnatal period.
- Refer to your local Adult Venous Thromboembolism (VTE) Prevention guidelines.
|
|---|
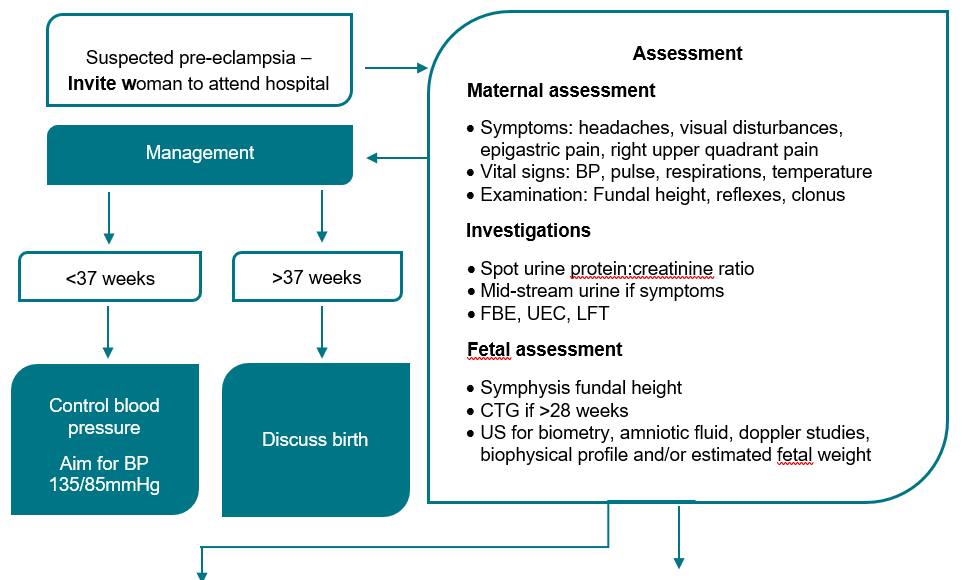
Pre-eclampsia
Diagnosis of pre-eclampsia
Most women with pre-eclampsia will have HT and proteinuria, but pre-eclampsia is also diagnosed in the setting of HT and any of the findings. (Appendix 3.)
Use of sFlt-1PIGF Ratio
Rule out: sFlt-1/PlGF ratio testing in ruling out pre-eclampsia is conditionally recommended by SOMANZ 2023 where a test result is available in a timely manner. A sFlt-1/PlGF ratio of ≤38 can be used to rule out pre-eclampsia within 1- 4 weeks of testing in women with clinical suspicion of pre-eclampsia.
Other roles: The use of the sFlt-1/PlGF ratio in diagnosing pre-eclampsia, determining fetal outcomes, severity of disease or timing of birth and its use in routine screening in asymptomatic women is not recommended until more data are available to support its use in these settings.
The use of the sFlt-1/PlGF ratio should not replace clinical assessment and management decisions should not be made based on the sFlt-1/PlGF ratio alone.
At present, timely access to this testing is not widely available throughout Victoria. Results can be obtained within 4-6 hours in centres with local testing capability and within a few days in centres where offsite testing is required.
Antihypertensive therapy | Pharmacological Management for stable Hypertension. (Chronic, gestational and non-severe pre-eclampsia) and Pharmacological Management for Severe Hypertension. - Medications may be required for stabilisation while awaiting transfer to a higher capability level service.
- Blood pressure target is ≤135/85 mmHg.
|
|---|
| Progression | - Clinical progression is unpredictable, so close clinical surveillance is required for all women with pre-eclampsia Increasing severity may be indicated by:
- difficult to control BP
- maternal symptoms
- deteriorating biochemical or haematological investigations
- eclampsia.
- Deteriorating fetal status -The definitive treatment is birth of the fetus and placenta.
- See Timing of Birth.
|
|---|
| Antenatal Care | - Care in the antenatal period depends on the gestation and severity of disease.
- Ensure ongoing obstetric review and medical review if required.
- Initial inpatient management is usually required, but there may be a role for outpatient management after senior review
- Maternal observations while an inpatient will depend on disease severity.
- See Vital signs as per Severe Hypertension.
- See Investigations as per Severe Hypertension.
|
|---|
| Referral | - If transfer is required, seek assistance from regional partners or PIPER.
- Refer women with severe early onset pre-eclampsia to a Level 6 Maternity Service – consult with PIPER as indicated.
|
|---|
| Venous thromboembolism (VTE) | - Pre-eclampsia is an independent risk factor for venous thromboembolism (VTE) Refer to your local Adult Venous Thromboembolism (VTE) Prevention guidelines.
|
|---|
| Fluid management | - Administration of intravenous fluids may contribute to a risk of pulmonary oedema. Consider IV fluid administration only after senior consultation.
- Hourly fluid balance and an indwelling urinary catheter maybe required.
- Diuretics are usually not recommended.
|
|---|
| Fetal neuroprotection | - Consider MgSO4 for fetal neuroprotection for planned birth less than 30 weeks gestation.
|
|---|
| Care in labour | |
|---|
| Postpartum | - Refer to Postpartum for management post birth.
|
|---|
Pharmacological Management for stable Hypertension (Chronic, gestational and non-severe pre-eclampsia)
- Oral medications such as labetalol, methyldopa and/or nifedipine can be used in managing stable hypertension in pregnancy. The choice of agent should be individualised based on the women’s clinical history and through a shared informed decision-making process.
- Oral antihypertensive treatment can safely be initiated in the outpatient setting for some women following a detailed assessment. The woman should be reviewed within 2-3 days to 1 week to assess response to therapy, progression of disease and need for escalation of therapy.
- Antihypertensives act via different pathways, hence it may be appropriate to introduce an additional agent if the blood pressure is not being adequately controlled with a single agent, rather than continuing to increase the dose to a maximal dose.
- Antihypertensive therapy is indicated if:
- systolic BP is ≥140 mmHg; and/or
- diastolic BP is ≥90 mmHg.
| Medication | Dose (all oral route) | Comments |
|---|
| Methyldopa | 250-750 mg Two to three times daily | - Alpha blocker.
- Slow onset of action over 24 hours.
- May cause dry mouth, sedation, dizziness, blurred vision.
- Use with caution in women with a history of depression.
|
| Labetalol | 100-400 mg Three to four times daily | - beta blocker.
- Contraindicated in women with asthma, chronic airway limitation.
|
| Nifedipine SR (slow release) | 20-60mg twice day Maximum dose is 120 mg daily | - Calcium channel blocker.
- Avoid in women with aortic stenosis.
- May cause peripheral oedema.
|
Good Practice Points - Oral hydralazine can be used as a second line agent.
- Angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers are contraindicated in pregnancy but are safe to use postpartum in breastfeeding women.
| |
Pharmacological Management for Severe Hypertension
- Oral antihypertensives may be appropriate as a first line. However, if an appropriate response is not achieved within a 30-45-minute timeframe or if the SBP ≥ 180 mmHg, intravenous therapy is likely to be more effective. The recommended first line agent (in the absence of contraindications) is Labetalol (Appendix 4.)
- When administering intravenous antihypertensive, an obstetric emergency trolley available, may be helpful.
- Aim for a BP no lower than 130mmHg systolic and 80mmHg diastolic. Co-administration of longer acting oral agents may assist with achieving a sustained response.
- Caution should be exercised to avoid hypotension as this may contribute to fetal compromise. Continuous fetal monitoring should be instituted when administering therapy for severe hypertension according to gestational age.
- Persistent or refractory severe hypertension may require repeated doses.
- Response to an agent should be assessed over 30-45 minutes before additional agents are added, however it should be noted that the maximal dose of an agent does not need to be achieved before considering the addition of a second agent.
| Medication | Dose | Route | Comments |
|---|
| Nifedipine immediate release | 10-20 mg capsule | Oral | - Repeat after 30 minutes.
- Maximum dose: 45 mg in 24 hours.
|
| Labetalol | 20-80 mg | Slow IV bolus over 2 minutes | |
| Hydralazine | 5-10 mg | Slow IV bolus over 3-5 minutes | |
Magnesium Sulphate (MgSO4) for seizure prophylaxis
- Prophylactic MgSO4 is strongly recommended in women at risk of eclamptic seizures or recurrent eclampsia (Appendix 6.)
- Seizures may occur antenatally, intra-partum or postpartum, usually within 24 hours of delivery but occasionally up to 7 days later
- Consider the use of prophylactic MgSO4 in women with:
- persisting or resistant severe hypertension (≥160/110mmHg)
- features of neurological irritability. (ongoing or recurring severe headaches, visual scotomata, clonus, hyperreflexia)
Magnesium Sulphate (MgSO4) for fetal neuroprotection
- The use of magnesium sulphate for fetal neuroprotection in women with preeclampsia at risk of preterm delivery <30 weeks of gestation is strongly recommended. (Appendix 6.)
- The use of magnesium sulphate for fetal neuroprotection in women with preeclampsia at risk of delivery between 30-34 weeks of gestation should be individualised based on clinical assessment and through a shared informed decision-making process with the woman.
- The use of MgSO4 is recommended:
- regardless of the number of babies in utero
- regardless of the anticipated mode of birth
- whether or not antenatal corticosteroids have been given
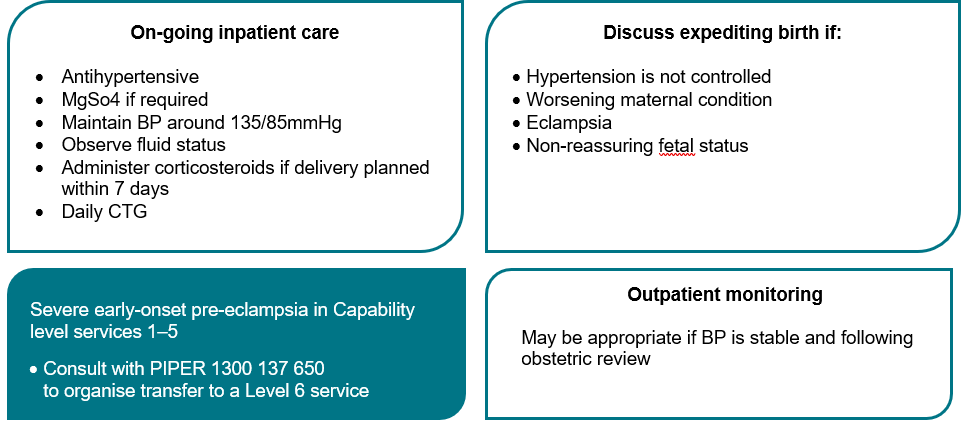
Eclampsia
- Eclampsia remains rare in Australia but is the presumed diagnosis in pregnant women who present with a seizure.
- MgSO4 is the recommended first line treatment.
- Consider alternative diagnoses including epilepsy, cerebral venous thrombosis, intracranial haemorrhage, meningitis, space-occupying lesions, metabolic disorders, head trauma and drug or alcohol-related issues.
- Eclamptic seizures are usually self-limiting.
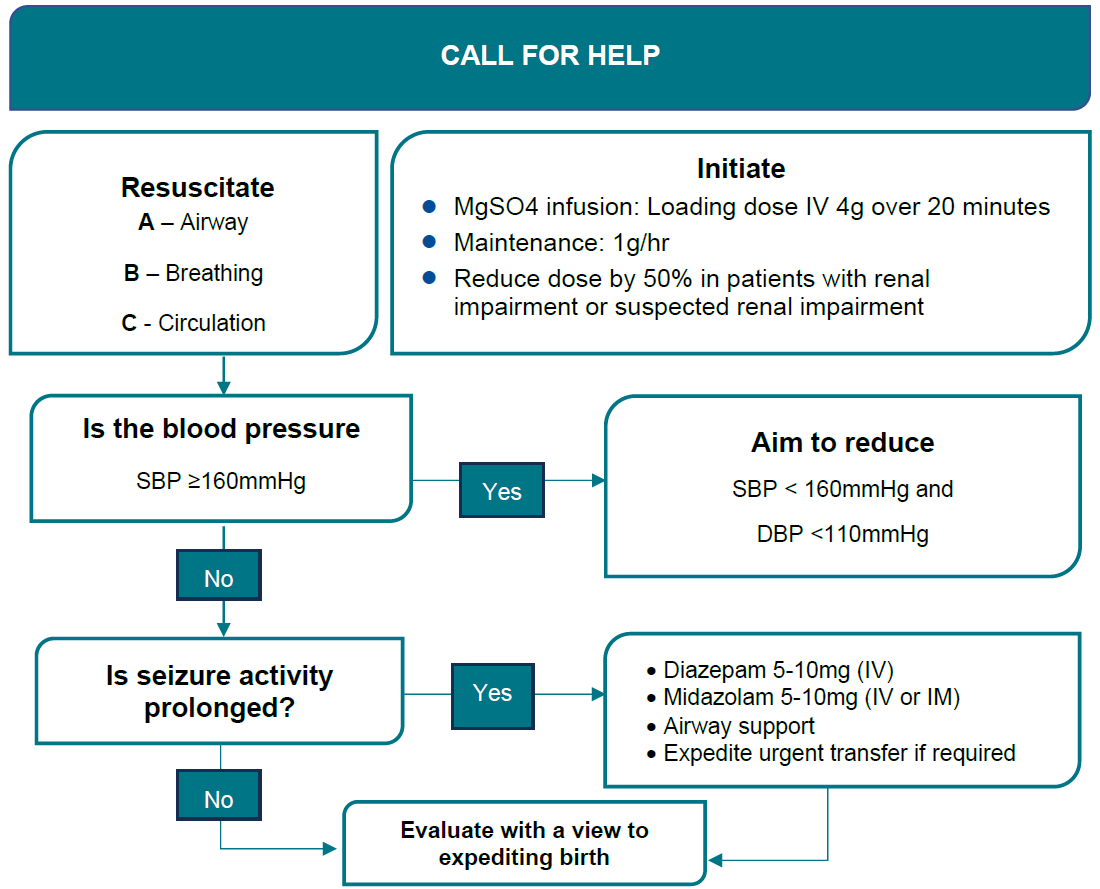
See Eclampsia flowchart >
| Goals of Treatment | - Terminate the seizure.
- Prevent recurrence.
- Control hypertension.
- Prevent maternal and fetal hypoxia.
|
|---|
Resuscitation | - Follow the basic principles of resuscitation.
- Call for help using emergency codes.
- Protect the woman from harm and move her to a left lateral position to decrease risk of aspiration.
- Ensuring a patent airway, oxygen by mask and intravenous access.
|
|---|
| Hypertension | - Control of severe hypertension to levels below 160/100 mmHg.
|
|---|
| Seizure control | - Intravenous MgSO4 Administration. (Appendix 6)
|
|---|
| Recurrent Seizures | - Where a seizure has not resolved within 2-3 minutes or recurs prior to commencing MgSO4, it is reasonable to administer one of the following agents intravenously over 2-3 minutes whilst commencing the MGSO4 infusion.
- Note: these agents are NOT first line treatment agents, nor can they replace MgSO4 infusion.
- Diazepam 5–10 mg IV.
- Clonazepam 1–2 mg IV.
- Midazolam 5–10 mg IV.
|
|---|
Birth | - Plan timing and mode of birth with senior staff once the woman’s condition is stable.
- Consult with Regional Partners /Paediatric team/PIPER to consider the most suitable place for birth.
- At preterm gestations, the differential diagnoses of eclampsia should be considered prior to proceeding with elective birth.
|
|---|
| Post seizure care | - Provide close clinical surveillance in an appropriately staffed area.
- Provide woman centred care including postnatal debriefing.
|
|---|
Timing of birth
Essential or gestational hypertension
- In the setting of stable maternal and fetal wellbeing, delivery should be considered between 37- and 39-weeks’ gestation
- Timing of delivery will need to consider the individual clinical scenario including comorbidities, blood pressure control and fetal wellbeing.
Pre-eclampsia
- Delivery is indicated at 37/40.
- Prior to 36+6 weeks, expectant management should be undertaken where maternal and fetal wellbeing are stable.
- Birth should occur earlier if maternal or fetal compromise is present – see table below ‘Indications for birth’.
- There is limited data to support the use of angiogenic biomarkers in determining timing and indication for birth and they should not generally be used for this purpose.
Indications for birth
Consult for further advice as required.
Maternal
- Neurological features. (such as eclampsia, severe intractable headache or repeated visual scotomata)
- Repeated episodes of acute (severe) hypertension despite maintenance treatment with multiple antihypertensive agents.
- Pulmonary oedema.
- Thrombocytopenia.
- Abnormal or rising serum creatinine.
- Rising liver enzymes.
- Hepatic rupture.
- Abruption.
Fetal
- Non-reassuring fetal status (or fetal demise) either clinically, on CTG or ultrasound parameters.
Care in Labour: Pre-eclampsia, essential or gestational hypertension
| Aspect | Consideration |
|---|
| Multidisciplinary approach | - Consult early with a multidisciplinary including obstetric midwifery and anaesthetics
- Care to be provided in Birthing with one-to-one midwifery care.
|
|---|
| Mode of birth | - If ≥ 34 weeks gestation, vaginal birth is preferable, if possible.
- If <32 weeks' gestation, the success of induction is reduced and a caesarean section is likely to be necessary.
- If vaginal birth is planned and the cervix is unfavourable, consider cervical ripening.
|
|---|
| Observations | - Blood pressure, pulse, respiratory rate, oxygen saturations and conscious state every 15 minutes until stable.
- Refer to your local guideline/SCV Guideline - Care in Labour.
- Assess the woman for and report immediately, any of the following signs or symptoms:
- altered mental state
- sudden rise in BP or BP≥160/110mmHg
- oliguria, (urine output <80 mL in 4 hours)
- oxygen saturations <94%
- persistent frontal headache
- visual disturbances
- epigastric or right upper quadrant pain
- hyperreflexia
- sustained clonus.
|
|---|
| Antihypertensive therapy | - Women should continue to receive their prescribed oral antihypertensive therapy during labour if hypertension is not adequately controlled during labour with oral antihypertensives, intravenous therapy may be required.
- For women receiving Labetalol intravenously. (Appendix 4)
- For women receiving hydralazine intravenously. (Appendix 5)
|
|---|
| Fluid management in: mild pre-eclampsia, essential hypertension or gestational hypertension | - Fluid balance charting.
- Caution is advised with IV fluids.
- Insert large bore intravenous cannula – more than 1 may be required if a MgSO4 infusion is used x2.
- Prevention or treatment of hypotension with drugs such as ephedrine, phenylephrine or metaraminol is effective and appears safe in preeclamptic women if required but can cause rebound hypertension.
|
|---|
| Fluid management in severe pre-eclampsia | - Insert large bore intravenous cannula – more than 1 may be required if a MgSO4 infusion is used.
- Caution with use of iv fluids.
- Strict fluid balance charting.
- Fluid loading is not usually essential prior to epidural anaesthesia.
- Restrict total fluid intake including intravenous therapy to 80mL/hour.
- Where oxytocin is used for labour induction or augmentation, use low volume dosing of oxytocin infusion.
- Insert an indwelling urinary catheter with urometer and measure urine hourly.
- Observe for oliguria - <80mL /4 hours.
- Observe for pulmonary oedema.
|
|---|
| Eclampsia prevention | - See MgSO4 for seizure prophylaxis. (Appendix 6)
|
|---|
| Oral intake | - Refer to your local care in labour or SCV Care in labour guidelines.
- If at risk of an eclamptic seizure and/or emergency caesarean section during labour, restrict intake to clear fluids or nil orally.
|
|---|
| Fetal monitoring | - Continuous CTG if ≥28 weeks. (or at consultant discretion at earlier gestations)
|
|---|
| Analgesia in labour | - Regional analgesia may be preferred to manage blood pressure.
- Discuss with anaesthetic team regarding platelet thresholds.
- Refer to your local Epidural Analgesia in labour guidelines.
|
|---|
| Second stage of labour | - Do not routinely limit the duration of the second stage of labour if BP is within target range - refer to SCV Care in Labour guideline for management of second stage.
- Consider operative birth in the second stage of labour for women where hypertension has not responded to initial treatment.
|
|---|
| Third stage of labour | - Active management of the 3rd stage with 10mg of Oxytocin by IM or IV injection. Refer to SCV Care in Labour policy Management of the Third Stage of Labour guideline
- Avoid Syntometrine/ Ergometrine in women with hypertension.
|
|---|
| Additional investigations | - Consider placental histopathology if earlyonset pre-eclampsia.
|
|---|
Post-Partum Management
| Context | - Hypertensive disorders of pregnancy may develop for the first time during the postpartum period.
- Postnatal care should be individualised according to the severity of pre-eclampsia and the rate of resolution.
|
|---|
| Place of care | - Where MgSO4 has been given for seizure prevention, the infusion should usually continue for 24 hours after birth or the last eclamptic seizure (whichever is the later) in Critical Care Unit or in Birth Suite
- Transfer to the postnatal ward only after consultation with senior obstetric staff.
|
|---|
| Observations on the postnatal ward | - Continue close monitoring.
- Record observations on the Maternity Observations chart until:
- BP is stable
- urine output has normalised
- blood investigations are stable or improving
- Ask woman if she has symptoms such as severe headache and epigastric pain.
- Accurate fluid balance charting may be required for women with moderate or severe disease using an indwelling urinary catheter with hourly urometer. Observe for oliguria - <80 mL/4 hours.
- Reduce frequency of monitoring only after discussion with the treating obstetric/medical team.
|
|---|
| Investigations | - Reassessment of proteinuria is not required.
- Women requiring ongoing treatment with MgSO4 may need reassessment of blood tests in the first 24 hours following birth.
- For women not requiring MgSO4, who have clinical resolution and antenatal blood tests were normal, further investigations are not required.
- If haematological or biochemical abnormalities do not resolve, consider further specific investigations.
|
|---|
| Escalation of care | - Postpartum Hypertension with systolic BP >160mmHg and /or diastolic BP >110mmHg requires escalation of care to Obstetric Consultant, GP Obstetrician, Regional Partners or PIPER.
|
|---|
| Medication | - If hypertensive (SBP >140 mmHg or DBP > 90 mmHg), consider commencement/continuation of antihypertensive therapy.
- The agents recommended for antenatal treatment can be used for the postnatal treatment if breastfeeding.
- Consider therapy with enalapril to reduce dosing frequency.
- If women are NOT breastfeeding, standard anti-hypertensive therapy can be used.
- For women prescribed beta blockers, refer to your local Neonatal Hypoglycaemia for BGL monitoring of the neonate.
- Non-steroidal anti-inflammatory drugs (NSAIDs) should be avoided in the immediate post-partum period.
- In the absence of an alternative analgesic agent, the use of NSAIDs should be limited to short-term inpatient usage.
|
|---|
| VTE | - Assess for risk of VTE and institute non-pharmacologic and pharmacologic prophylactic measures if required.
- Refer to your local Adult Venous Thromboembolism (VTE) Prevention guidelines.
|
|---|
| Discharge & Collaborative Care | - Suitability for discharge to community care should consider blood pressure control, evidence of resolving biochemical derangement and usual postpartum issues.
- Women with a blood pressure reading of <150/100mmHg or lower are suitable for discharge and follow-up in the community.
- Consider the risk of late seizures and the peak postpartum BP when timing discharge.
- Domiciliary midwife should review all women with hypertensive disorders after discharge.
- Send a discharge summary to the GP and MCHN reflecting the pregnancy complications, management and specific recommendations for follow up.
- Women with new onset hypertension during pregnancy who are discharged on antihypertensives or with persisting hypertension should be advised to have their blood pressure checked weekly or fortnightly with the GP, depending on blood pressure levels, to allow reduction and cessation of antihypertensives when possible.
- Women with pre-eclampsia with severe features (e.g., early onset pre-eclampsia or evidence of significant end organ effects (eclampsia, stroke etc) may benefit from postnatal follow up with the obstetric or obstetric medicine team.
|
|---|
| Long term post-partum care and future pregnancies | - Postnatal counselling should include a discussion about:
|
|---|
References
- Society of Obstetric Medicine Australia and New Zealand. Hypertension in pregnancy guideline. Sydney; 2023. Available from: https://www.somanz.org/content/uploads/2024/01/SOMANZ_Hypertension_in_Pregnancy_Guideline_2023.pdf [Accessed April 2024]
- RANZCOG. Intrapartum fetal surveillance: Clinical guideline- 4th ed. East Melbourne, VIC: RANZCOG; 2019. Available from: https://ranzcog.edu.au/RANZCOG_SITE/media/RANZCOGMEDIA/Women%27s%20Health/Statement%20and%20guidelines/Clinical-Obstetrics/IFSGuideline-4thEdition-2019.pdf?ext=.pdf
- National Institute for Health and Clinical Excellence (NICE). Hypertension in pregnancy: diagnosis and management. Clinical Guideline 133. [Internet]. 2019 [Accessed 2024 April 15]. Available from: http://www.nice.org.uk
Acknowledgements
- Mercy Health - IV Labetalol for Management of Severe Hypertension in Pregnancy Procedure - Nov 2020 Monash Health - Hypertensive disorders in pregnancy, pre-eclampsia, eclampsia Clinical Guideline - Jan 2024
- Royal Women’s Hospital - Hypertension - Management of Acute - Guideline - Dec 2023
- Western Health - Intravenous Labetalol Administration for Management of Severe Hypertension in Pregnancy or Postpartum - Feb 2021
- Western Health - Management of Hypertension in Pregnancy – Pre-eclampsia and Eclampsia - Feb 2023
Citation
To cite this document use: Safer Care Victoria. Hypertension in Pregnancy Guideline [Internet]. Victoria: Maternity eHandbook; 2024 [cited xxxx] Available from: https://www.safercare.vic.gov.au/clinical-guidance/maternity
Appendix 1. Assessment of Proteinuria
| Protein Assessment | At booking: - If proteinuria is present on urine dip stick, or the woman has renal disease, or risk factors for renal disease eg SLE, chronic hypertension, perform a spot urine protein: creatinine ratio. (PCR)
During pregnancy: - Spot urine PCR greater than 30 mg/mmol is diagnostic of proteinuria in pregnancy (in the absence of an alternative explanation. (e.g., UTI)
- Once proteinuria has been detected, there is no established role for serial testing.
- 24-hour urine collection is no longer used.
- Absence of proteinuria does not exclude a diagnosis of pre-eclampsia. (Appendix 3)
|
|---|
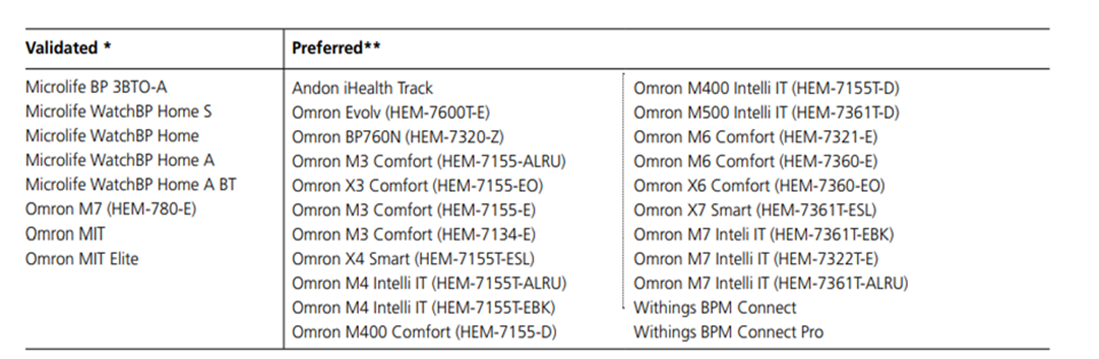
Appendix 2. Home BP monitoring
- Where appropriate, home blood pressure monitoring (HBPM) with the use of a validated blood pressure device can be utilised in women with chronic or gestational hypertension.
- The use of HBPM, however, should not replace the minimum recommend frequency of antenatal review according to the woman’s parity and stage of pregnancy.
- Women should be provided with written information about technique and when to seek help.
- Compliance and technique with home blood pressure monitoring should be reassessed at each review to ensure ongoing suitability.
Home BP machines validated in pregnancy
Appendix 3. Diagnosis of Pre-eclampsia
Most women with pre-eclampsia will have HT and proteinuria, but pre-eclampsia is also diagnosed in the setting of HT and any of the findings listed below. (Hypertension may not be the predominant clinical feature)
The use of the sFlt-1/PlGF ratio in diagnosing pre-eclampsia, is not currently recommended
| Organ/System | Features |
|---|
Renal | - Random urine protein to creatinine ratio greater than or equal to 30 mg/mmol7 from an uncontaminated specimen.
- Serum or plasma creatinine greater than or equal to 90 micromol/L. or
- Oliguria (less than 80 mL/4hours or 500 mL/24 hours).
|
Haematological | - Thrombocytopenia. (platelets under 150 x 10/L)
- Haemolysis. (schistocytes or red cell fragments on blood film, raised bilirubin, raised lactate dehydrogenase (LDH), decreased haptoglobin)
- Disseminated intravascular coagulation. (DIC)
|
| Liver | - New onset of raised transaminases (over 40 IU/L) with or without epigastric or right upper quadrant pain.
|
Neurological | - Headache.
- Persistent visual disturbances. (photopsia, scotomata, cortical blindness, retinal vasospasm)
- Hyperreflexia with sustained clonus.
- Convulsions. (eclampsia)
- Stroke.
|
| Pulmonary | |
Uteroplacental | - Fetal growth restriction. (FGR)
- FDIU.
|
Appendix 4. IV Labetalol regime
| Labetalol for treatment of severe hypertension during pregnancy or the postpartum period |
Resources required | - One to one midwifery care in birthsuite or highdependency unit for the duration of therapy
- IV access should be secured with two large bore IV cannulas (16g)
- Consider triple lumen connection port
- A fluid bolus of sodium chloride 0.9% or compound sodium lactate (Hartmann’s solution) is not necessarily required prior to labetalol administration but may be considered according to the patient’s fluid status.
|
| Contraindications | - Severe or uncontrolled asthma.
- Uncontrolled congestive cardiac failure.
- History of allergic disorders with a predisposition to bronchospasm.
- Second- or third-degree atrioventricular block. (AV block)
- Sick sinus syndrome. (without pacemaker)
- Shock. (cardiogenic or hypovolaemic)
- Bradycardia with a heart rate <60bpm.
|
| Bolus dose IV labetalol |
| Administration | - Bolus doses can be administered:
- Using a controlled infusion device over 2 minutes; Note: This is the preferred method of administration
- Or
- Manual IV injection over 2 minutes
- Note: An obstetric registrar/consultant or GPO must remain present during ALL bolus doses of labetalol.
|
| Syringe preparation | - Controlled infusion device.
- The line is required to be primed with labetalol prior to loading the syringe into the controlled infusion device and connecting to the giving set.
- Label the line with ‘Labetalol’.
- Each individual bolus dose is drawn up and administered via a separate syringe.
- Ensure only the prescribed dose for the bolus dose is loaded into the syringe driver pump.
- Label syringe with ‘Labetalol (5 mg/mL)’.
- Load syringe into syringe driver pump.
- Connect to cannula.
- Manual IV injection.
- Withdraw only the required dose for each individual bolus dose undiluted from the ampoule.
- Label the syringe as above.
|
| Maximum bolus doses | - Up to two bolus doses may be administered after which a labetalol infusion should be commenced if the blood pressure remains above the target range (systolic BP≥160mmHg and / or diastolic BP ≥110mmHg).
- The maximum daily dose of labetalol should not exceed 300 mg in 24 hours.
|
| Requirements | - Bolus doses of IV labetalol must be administered under the direct supervision of medical staff, whether via syringe driver or manual bolus.
- The woman should be nursed in supine with lateral tilt, lateral position or semi-recumbent during and up to three hours after IV labetalol administration due to the potential side effects of orthostatic hypotension.
- CTG monitoring should always be undertaken for pregnant women >28 weeks receiving labetalol boluses.
- The maximum daily dose of labetalol should not exceed 300 mg in 24 hours.
|
| Bolus dose | - 20mg (4mL) administered by slow IV injection over two (2) minutes.
- One further bolus can be administered at 10 minutes if necessary.
- Syringe Driver/controlled infusion device.
- Follow your local guidelines on how to administer the bolus dose via your local pump/syringe driver device.
OR - When performing a manual IV injection:
- flush cannula with 10 mL sodium chloride 0.9%
- inject 20 mg (4 mL) over 2 minutes
- flush cannula with 10 mL sodium chloride 0.9%.
|
| Vital signs | Check vital signs: - Every 5 minutes for 15 minutes.
- Then every 15 minutes for 1 hour.
- Then every 30 minutes for 3 hours.
- Continuous CTG for a minimum of 3 hours after completion of bolus dose.
|
| IV labetalol infusion |
| Administration | - All infusions to be administered using a syringe driver pump or large volumetric pump with syringe adapter.
- IV labetalol infusion should be commenced if the blood pressure remains elevated (systolic BP≥160mmHg and / or diastolic BP ≥110mmHg) after three bolus doses.
- As individual responses to labetalol will vary, medical staff must determine the infusion rate.
- Infusion rates will vary from 20 mg/hour (4 mL/hour) to 160 mg/hour (32 mL/hour).
|
| Syringe Preparation | - 200mg in 40mL (5mg/mL) drawn up undiluted into a 50mL syringe.
- Label syringe with ‘Labetalol 200 mg in 40 mL (5 mg/mL)’.
- If not already primed, ensure the line is primed with labetalol prior to commencing infusion.
- Follow your local guidelines on how to administer the bolus dose via your local pump/syringe driver device.
|
| Commencing infusion | - Commence at 20 mg/hour (4 mL / per hour): Infusion may be used up to 160mg/hour.
- Follow your local guidelines on how to administer the bolus dose via your local pump/syringe driver device.
- Confirm 200 mg in 40 mL.
- Input VTBI: 40 mL.
- Input rate: 4 mL/hr. (dose 20 mg/hr)
- Ensure line is labelled appropriately.
|
| Titrating infusion | - Infusion to be titrated according to blood pressure every 15 minutes.
- A medical order is required for each rate change.
- Discontinue by weaning over 1-2 hours when blood pressure is consistently less than 155 / 95 mmHg.
- In some clinical scenarios, ceasing without weaning may be more appropriate.
- If the systolic blood pressure consistently <140mmHg or diastolic <90mmHg, notify medical staff regarding cessation of infusion and review.
|
| Observations | - Use Maternity Track and Trigger Observations chart or high dependency chart.
- BP Every five minutes for 15 minutes after commencement of an infusion then every 15 minutes.
- HR 30 minutes.
- Temperature 2 hourly.
- Continuous CTG >28 weeks gestation, while on treatment.
|
| Requirements | - The BP should be monitored every 15 minutes throughout an infusion.
- The woman should be placed supine with lateral tilt, lateral position or semi-recumbent during and up to three hours after IV labetalol administration, due to the potential side effects of orthostatic hypotension.
- The maximum daily dose of labetalol should not exceed 300 mg in 24 hours.
|
| Postpartum Treatment | - IV labetalol infusions can be used until oral treatment can be commenced
- Breastfeeding:
- Labetalol is considered safe to use in breastfeeding, observing for signs of hypotension and/or bradycardia.
|
Appendix 5. Hydralazine regime
| Indication | - Acute control of severe hypertension
|
|---|
Contraindications | - Known hypersensitivity.
- SLE.
- Severe tachycardia. (greater than 125 bpm)
- Myocardial insufficiency.
- Right ventricular heart failure.
|
Precautions | - Suspected/confirmed coronary artery disease.
- Renal impairment.
- Hepatic impairment.
- Cerebrovascular disease.
|
| Route | |
| Bolus dose Syringe preparation | - Reconstitute the hydralazine 20 mg vial with 1 mL of water for injection to make a 20 mg/mL solution.
- Dilute 1 x 20 mg/mL reconstituted ampoule of hydralazine with 19 mL sodium chloride 0.9% (to obtain 1 mg per mL) in a 20 mL syringe. (final volume is 20 mL)
- Label as ‘Hydralazine 1mg per ml’.
|
Intermittent IV bolus | - The bolus dose is 5mg.
- The IV bolus is given by slow intravenous injection (pushed by hand) over 3-5 minutes.
- The initial response should occur within 5-15 minutes.
- Dose can be repeated every 20 minutes to a maximum of 3 bolus doses, each drawn up in a separate syringe.
- Commence a hydralazine infusion if the BP is still outside of target range following 3 x 5mg boluses.
- Maximum cumulative dose is 30 mg over24 hours.
|
| Continuous infusion Syringe preparation | - Dissolve 40 mg hydralazine powder (2 ampoules) in 2 mL of water for injections in a 50ml syringe. (40 mg in 2 mL)
- Add 38 mL of sodium chloride 0.9% to syringe 40 mg in 40 mL. (1 mg in 1 mL)
- Administer via syringe pump.
|
Continuous IV infusion | - Loading dose: Administer hydralazine at 1mg/min (i.e. 1mL/min of the prepared solution) for 10 minutes.
- Titrate infusion by 1 mg/hour, every 20 minutes until the optimum BP is achieved or the maximum rate is reached. (5mg/hour)
- If the blood pressure has reached the target range after 5 minutes administration of the loading dose (i.e. 5mg), the loading dose may be ceased.
- Maintenance dose: Continue infusing hydralazine at 1-10mg/hr (1-10mL/hr) to maintain a diastolic BP of 90 - 100mmHg.
|
Side effects | - Tachycardia.
- Headache.
- Flushing.
- Palpitations.
|
| Monitoring during bolus doses | - Monitor BP, pulse and oxygen saturations:
- every 5 minutes during administration and until stable,then
- hourly for 4 hours
- Continuous CTG if ante/intrapartum.
|
| Monitoring during infusion | - Monitor BP every 15 minutes untilstable, then;
- Hourly for duration of infusion. (unlessotherwise instructed)
- Continuous CTG if ante/intrapartum.
|
Additional considerations during monitoring | - Maintain strictfluid balance monitoring
- Monitor, as clinically appropriate:
- full blood count
- liver function test
- urea and electrolyte levels
- coagulation profile.
|
Appendix 6. MgSO4 for pre-eclampsia, eclampsia or fetal neuroprotection
Resources required | - One to one midwifery care in birth suite or high dependency unit for the duration of therapy.
- Dedicated IV line for MgSO4 using a controlled infusion device.
- Resuscitation support available.
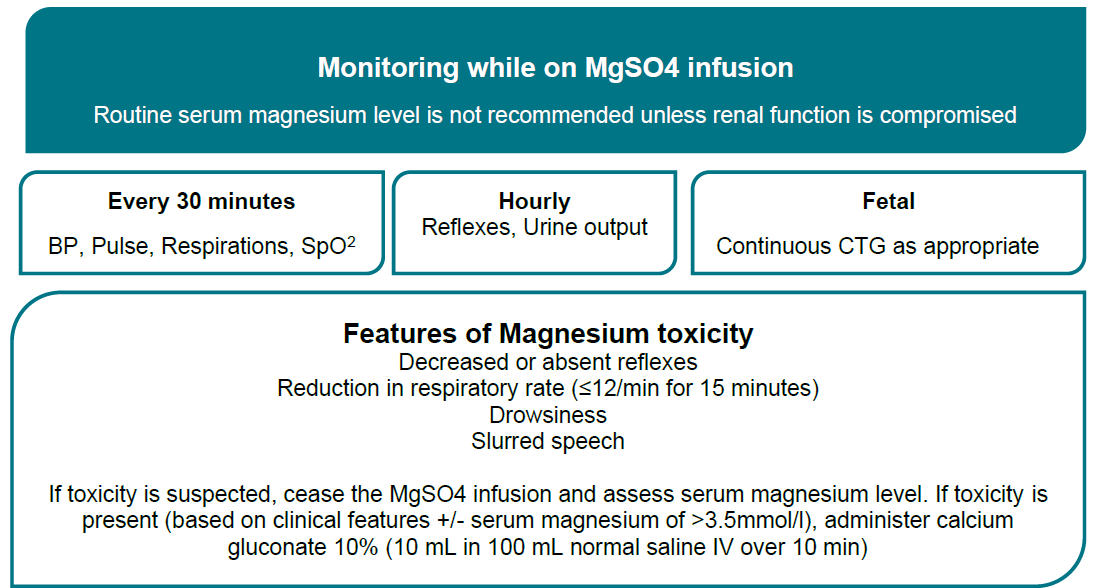
- Calcium gluconate 10% (0.22 mmol/mL) 10 mL vial available in case of respiratory depression/overdose.
|
|---|
| Contraindications | - Maternal cardiac conduction defects. (heart block)
- Hypermagnesaemia.
|
|---|
| Precautions | - Myasthenia gravis.
- If impaired renal function, monitor plasma magnesium levels.
|
|---|
| Loading dose | - 4g (8ml) is given over 20 minutes at a rate of 24mL/hour via controlled infusion device.
|
|---|
Maintenance dose | 1 g/hour (2ml/hr) IV for 24 hours. If impaired renal function: - reduce loading dose to 2g over 20 minutes and maintenance infusion rate to 0.5 g/hour.
|
|---|
Seizures | If new onset seizure or persistent seizures during MgSO4 infusion. (loading or maintenance) - give a further 2 g IV over 5 minutes
- if seizures persist, may be repeated in a further 2 minutes.
|
|---|
Side effects | - Related to hypermagnesaemia.
- Common (greater than 1%): nausea and vomiting, sensation of heat/flushing.
- Infrequent (0.1–1%): headache, dizziness.
|
|---|
Baseline observations | - BP, pulse, respiratory rate, level of consciousness.
- Oxygen saturation. (SpO2)
- Deep tendon reflexes.
- If antepartum: FHR/CTG depending on gestation.
|
|---|
Monitoring during loading dose | - BP, pulse and respiratory rate every 5 minutes (for minimum 20 minutes) until stable.
- Continuous SpO2.
- Continuous CTG if greater than 28+0 weeks gestation.
- Under 28 weeks' gestation, individualise assessment of fetal wellbeing.
- Observe for side effects.
- Check deep tendon reflexes (patellar; if epidural insitu, biceps) after completion of loading dose, notify obstetrician if absent and do not commence maintenance dose.
|
|---|
Monitoring during maintenance infusion | - BP, pulse, respiratory rate and SpO2 every 30 minutes.
- Temperature every 2 hours.
- Continuous CTG if greater than 28+0 weeks gestation.
- Under 28 weeks gestation, individualise assessment of fetal wellbeing.
- Strict fluid balance monitoring and documentation.
- indwelling urinary catheter is recommended.
- Hourly urine measurements - Observe for oliguria - <80mL /4 hours.
- Hourly Deep tendon reflexes.
- Record as: A=Absent, N=Normal, B=Brisk.
- If renal function is normal, serum monitoring is not required.
- Therapeutic serum magnesium levels are 1.7–3.5 mmol/L.
|
|---|
Urgent medical review | - Respiratory rate less than 12 breaths/minute.
- Absent deep tendon reflexes.
- Urine output less than 80 mL/4 hours.
- Magnesium serum levels greater than 3.5 mmol/L.
- Use Maternity Observations chart.
|
|---|
| Ceasing therapy | - Where MgSO4 has been given for seizure prevention, the infusion should usually continue for 24 hours after birth or the last eclamptic seizure.
- Before discontinuing therapy, ensure clinical improvement of blood pressure and diuresis is evident and condition is stable.
|
|---|
Flowchart - Hypertension diagnosed after 20 weeks gestation

Flowchart - Pre-eclampsia: Assessment and Management
Flowchart – Eclampsia Management